Executive summary
With the increase in the use of artificial intelligence (AI) in pharmaceutical drug development, the race is on to develop life-saving drugs faster and more cost-efficiently. Companies in this space purport to help expedite time to market, but depending on cancer types, processes and available tumor samples, it’s rarely an easy solve.
Predictive Oncology’s Proof of Concept (PoC) Study reveals a more powerful and reliable tool that provides a competitive edge to oncology drug developers. The study tested the PEDAL platform’s ability to make drug response predictions. PEDAL, from Predictive Oncology (POAI), is a unique combination of AI technology, drug response data and a biobank of over 150,000 tumor samples for 137 tumor types.
The current early drug discovery process involves testing drug candidates against immortalized cell-lines and mice models with single-patient tumor xenografts. In this PoC, Predictive Oncology performed hands-on testing of 175 FDA-approved drugs using 130 ovarian tumor samples. PEDAL’s machine learning model based its drug-response predictions on the results from 720 hands on experiments and made an additional 4,600 drug/tumor type predictions—all with an accuracy rate of 92% and in only 11 weeks.
These results have strong implications not only for the new drug discovery process, but also in terms of identifying opportunities for drug repurposing. This PoC demonstrates that PEDAL is an efficient and cost-effective tool to expand an indication for an existing or abandoned drug using real tumor samples—not immortalized cell lines.
Study highlights
- The PEDAL platform assessed 175 existing, FDA-approved oncology drugs against 130 ovarian tumor samples.
- Researchers ran PEDAL’s AI-driven experimentation engine to iteratively improve the predictive model until results stabilized from round to round.
- The results from the PoC were subsequently validated. The validation work shows that PEDAL can predict whether or not a compound would be a hit for a given tumor sample with 92% accuracy.
- PEDAL completed actual lab experiments for only 3.16% of the experimental space and made high-confidence predictions for an additional 20.47% of all possible combinations.
- A number of potentially useful predictions were made regarding specific drug classes and their expected effectiveness across different patient tumors.
- This PoC demonstrates the power of PEDAL to make higher-confidence predictions, so researchers can select the best drug/tumor type combinations to increase their probability of technical success
Objective and background
The power of PEDAL (which stands for Patient-centric Discovery by Active Learning) comes from a unique combination of three exclusive assets:
- The world’s largest proprietary biobank of tumor samples—150,000+ clinical cases covering 137 different tumor types (plus associated assay capabilities).
- A supplementary knowledge base—historical drug response, biomarker, tissue imaging, patient characteristics and additional public data sets.
- Advanced AI machine-learning platform CORE™ (Computational Research Engine), developed by top researchers at Carnegie Mellon University and licensed exclusively to POAI; CORE takes a polypharmacological/pharmacogenomic approach to active machine learning, constructing models of all possible combinations of patient-specific drug response and using these models to efficiently drive rounds of wet-lab drug-response testing in an
iterative manner.
Objective
Demonstrate that the PEDAL system is a highly accurate, efficient and powerful tool with which to make high-confidence drug-tumor pairing predictions.
Methodology
The PEDAL proof of concept study was carried out in an experimental space of 175 FDA-approved drugs and 130 primary samples from ovarian tumors. The study consisted of two phases, the first of which demonstrates that PEDAL’s predictive model efficiently reveals drug-response patterns that provide insight into the treatment of ovarian cancers. The second phase focused on the wet-lab validation of the
accuracy of the predictions.
The study leveraged historical drug response data, biomarker data and general tumor histopathology categories of the tumor samples, and focused on learning which drugs effectively inhibit tumor cell growth.
Part 1: AI-driven experimentation
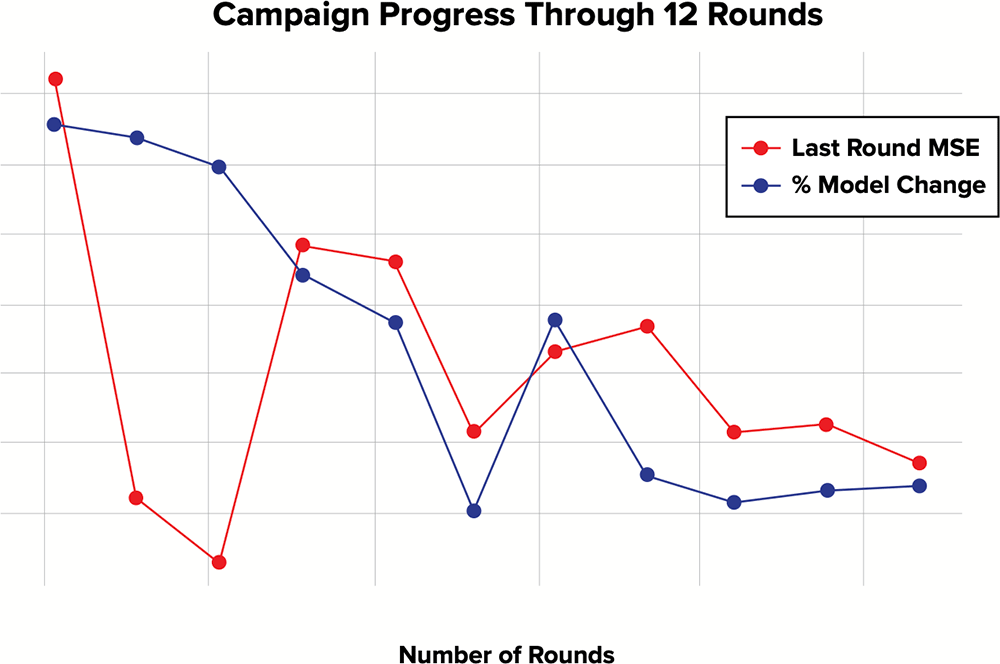
Figure 1. Campaign progress measures
Before the predictive modeling process begins, criteria are set to determine at what point a model has reached stabilization. These criteria are designed to meet the specific study needs and are actively calculated and graphed from round to round, as seen here.
The proof of concept study began with the AI machine-learning phase, in which the CORE platform performed the first round of predictive models for all possible combinations based on the features data available for 130 tumor samples and 175 drugs. After each round of modeling, CORE determined the most informative wet-lab testing to conduct. The results of these experiments were incorporated into the starting data for the next round of predictions in order to iteratively improve the predictive model until it stabilized (based on predetermined criteria). In the case of this study, stabilization occurred after
12 rounds (see figure 1), after which only 3.16% of the experimental space had been tested.
Experimentation highlights
• The team completed testing for 720 combinations of drug and patient tumor samples, and the resulting model made high-confidence predictions for an additional 20.47% of all possible combinations
• In a matter of weeks, PEDAL efficiently made over 4,600 high-level predictions of drug/tumor sample combinations
Part 2: Wet-lab validation
Upon completion of the PoC, additional wet-lab experimentation was used to validate the reproducibility and the accuracy of the high-confidence predictions generated by PEDAL.
Model accuracy results
Overall, the wet-lab validation revealed that the PEDAL platform was able to predict whether or not a compound would be a hit for a given tumor sample with 92% accuracy.
Results summary and implications
Through this PoC study, Predictive Oncology demonstrated that the PEDAL platform is a highly efficient tool to make confident predictions about drug-tumor pairings (up to 7x the number of drug-tumor-sample combinations actually tested in the lab). In addition to efficient identification of drug-tumor-type combinations, the platform has been validated to perform these predictions with 92% accuracy. This has strong implications not only for the new drug discovery process, but also in terms of identifying opportunities for drug repurposing.
PEDAL is a powerful tool in the fight against cancer
There’s a lot at stake in the fight against cancer, and companies that work smarter will come out ahead. The
PEDAL platform is now commercially available and can help oncology drug development companies gain a
competitive edge:
- Bring primary tumor samples, from a variety of tumor types, earlier into the drug discovery process
- Make higher-confidence predictions to select the best drug/tumor type combinations to increase the
probability of technical success
- Reduce the timeframe and increase the agility of the drug discovery process
- Gain the ability to repurpose drugs faster and more accurately and improve the diversity of the drug
portfolio for a given cancer
Start a PEDAL pilot program today
Complete the form and our PEDAL expert will be in touch to discuss customized solutions for your needs.
News & resources
White paper validates highly reproducible drug response data. Results enable AI platform to model and predict patient outcomes on historical samples Leveraging more than 20...
A recent white paper released by Predictive Oncology’s highlights the challenge of late-stage clinical trial failures and the company’s ability to better navigate those obstacles...
Company expands AI/ML driven capabilities to pursue novel biomarker discovery to predict patient outcomes and drug response in oncology. Read our white paper below that...



















